The International Sharps Injury Prevention Society (ISIPS) is proud to announce the addition of two new members to their organization: Roncadelle Operations and Medicalock. These companies have joined forces with ISIPS in their mission to prevent sharps injuries and promote safe practices in the healthcare industry.
Roncadelle Operations, a leading provider of medical solutions, brings its retractable safety syringe to the table. This innovative syringe is designed to reduce the risk of needlestick injuries for healthcare workers. With a simple one-handed operation, the needle is safely retracted into the syringe after use, eliminating the need for manual recapping and reducing the risk of accidental punctures.
Medicalock, a pioneer in surgical instrument safety, has also joined ISIPS in its efforts. Their Surgilock system is an adhesive cross-linked polymer pad that securely holds both metallic and non-metallic instruments and prevents them from breaking the sterile field or landing on the floor. This technology has been proven to reduce sharps injuries and improve overall safety in the operating room.
“We are thrilled to welcome Roncadelle Operations and Medicalock to our organization,” says Ron Stoker, President and Executive Director of ISIPS. “Their commitment to promoting safe practices and preventing sharps injuries aligns perfectly with our mission. We believe that by working together, we can make a significant impact in reducing the number of injuries and infections caused by sharps in the healthcare industry and beyond.”
ISIPS is dedicated to providing education, resources, and support to healthcare professionals in their efforts to prevent sharps injuries. With the addition of Roncadelle Operations and Medicalock, ISIPS is confident in its ability to continue making strides in this important cause.
For more information on ISIPS and their new members, please visit their website at www.isips.org. Together, we can create a safer and healthier environment for healthcare workers and patients alike. For more information email ron@isips.org or 801-783-3817.


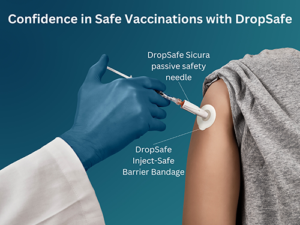


 be used it is important to make traditional needles as safe as possible.
be used it is important to make traditional needles as safe as possible.