Safety Product Category
Sharps Biohazard Spill Clean-up
Sharps Biohazard Spill Clean-up
Cleaning up bodily fluids such as blood, vomit, or urine isn’t just unpleasant—it presents a potential health hazard. it is important to clean up these potential health hazards without creating new ones for those kind individuals that end up cleaning up the mess.Sharps Biohazard Cleanup Kit and Disposal System
 Our Sharps Biohazard Spill Clean-Up Kit™ provides a safe way to properly handle biohazard substances.
Our Sharps Biohazard Spill Clean-Up Kit™ provides a safe way to properly handle biohazard substances.
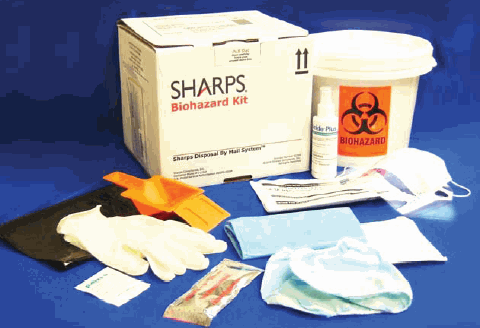
The Sharps Biohazard Spill Clean-Up Kit and Disposal System is the only complete solution for both cleanup and disposal by mail of bio-hazardous spills. It provides everything needed for the cleanup and disposal of biohazard fluids. It also provides a safe, simple, and regulatory-compliant method to remove these materials from your facility.
The cleanup kit, transportation, and destruction are all included in the price of the kit. The Sharps Biohazard Spill Clean-Up Kit is available in two convenient sizes to cleanup spills.
ADVANTAGES
- Compliance with federal, state and municipal regulations
- Only buy what you need and use as needed
- Elimination of expensive or unnecessary pick-ups by medical waste haulers
- No contract requirement
- No red bags in the trash
- Immediate dispose
- Transportation of waste via the U.S. Postal Service
SPECIFICATIONS
Each kit includes:
- 1 or 2-gallon mail-back bucket (sharps may be
placed into this bucket) - A protective plastic safety apron with sleeves
- 2 pair of protective non-latex gloves
- 1 Mask and face shield combination
- Protective hair and shoe covers
- 1 package of absorbent material
- Scraper and shovel
- 1 bottle of EPA-registered cleaner and disinfectant
- 1 hand cleaning towelette
- Absorbent towels
- 1 full set of instructions in both English and Spanish
- 1 Sharps Disposal by Mail packaging system (postage prepaid)
Item Number #51000 - Also available in a 2-gallon size





 permanently into place.
permanently into place.
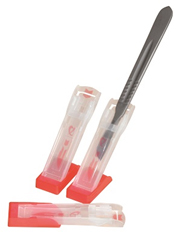
 A lightweight, unique, purpose designed, single-use device that provides a secure ‘hands free’ transfer medium for passing sharp instruments, thus reducing the risk of third party inflicted sharps injuries
A lightweight, unique, purpose designed, single-use device that provides a secure ‘hands free’ transfer medium for passing sharp instruments, thus reducing the risk of third party inflicted sharps injuries




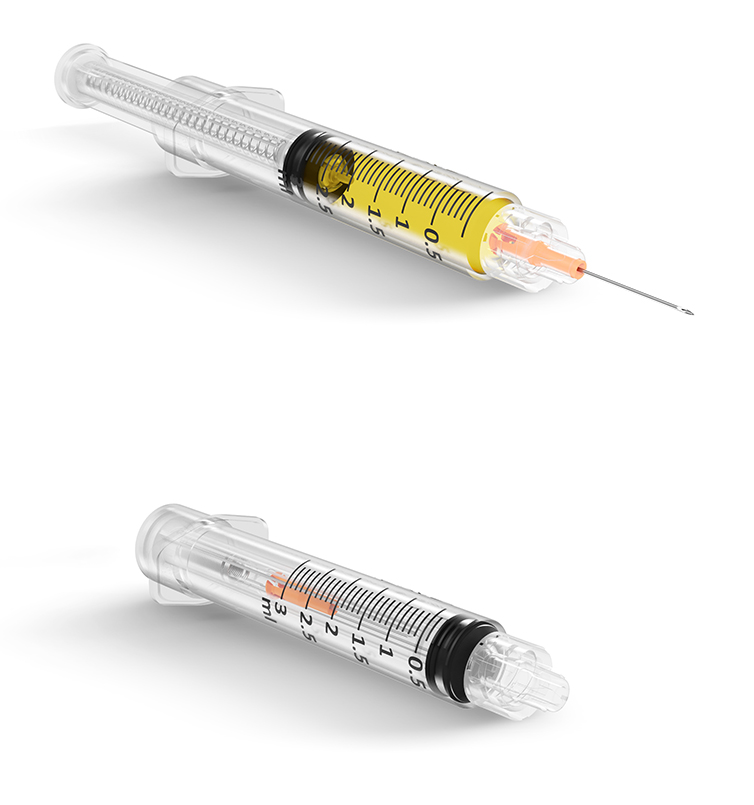
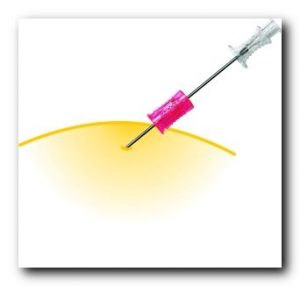
 VanishPoint syringes have been called “the gold standard for retractable needle syringes.”* They are available in a variety of sizes (0.5, 1, 3, 5, and 10mL), needle gauges, and needle lengths. The needle is automatically retracted directly from the patient into the barrel of the syringe when the plunger handle is fully depressed. The pre-removal, automated retraction virtually eliminates exposure to the contaminated needle, effectively reducing the risk of needlestick injury.
VanishPoint syringes have been called “the gold standard for retractable needle syringes.”* They are available in a variety of sizes (0.5, 1, 3, 5, and 10mL), needle gauges, and needle lengths. The needle is automatically retracted directly from the patient into the barrel of the syringe when the plunger handle is fully depressed. The pre-removal, automated retraction virtually eliminates exposure to the contaminated needle, effectively reducing the risk of needlestick injury.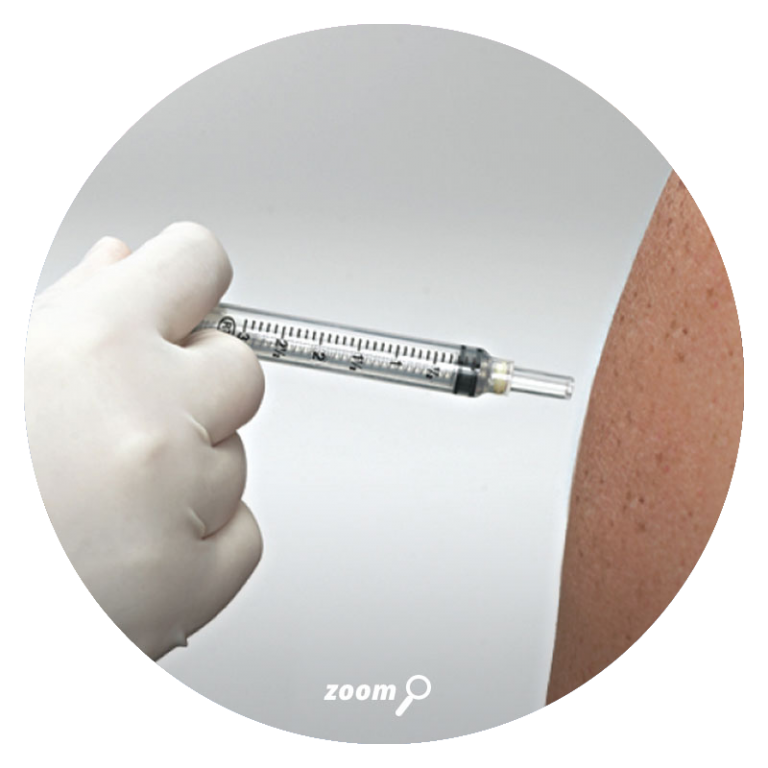
 (OSHA) bulletin states that8:
(OSHA) bulletin states that8: